 Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
Saturday, 28 April 2012
Dihydrogen monoxide ...
.. also known as water! It is the only substance naturally occurring on Earth that, simultaneously, is in three distinct states or forms – solid, liquid, and gas. For example on a cold winter's day, snow and ice can cover a field while water flows in a nearby river and clouds drift by, above in the sky. It is a liquid at standard temperature and pressure as well (luckily) as well as tasteless and odourless (in its pure form).
Water is one of the very few substances where the liquid form is heavier than the solid - thankfully - otherwise the oceans would freeze to the very bottom of the sea floor and the Earth would be a frozen planet without life. The maximum density of water occurs at 3.98 °C. It expands to occupy 9% greater volume in the solid state, which accounts for the fact of ice floating on liquid water, as in icebergs. Liquid form has a density is 1,000 kg/m3 liquid at 4 °C whereas solid (ice) has a density of 917 kg/m3).
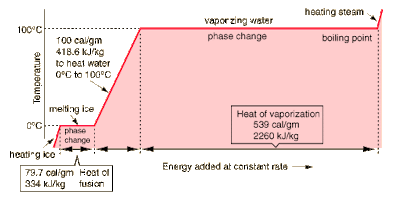
Another remarkable property of water is its extremely high capacity to absorb heat without a significant increase in temperature. Related to heat capacity is latent heat. Latent heat is the quantity of heat energy in BTU per pound or calories per gram absorbed or released by a substance undergoing a change in phase (liquid to solid and vice versa, and liquid to gas or vice versa) without a change in temperature. The latent heats of fusion and evaporation of water are unusually high as illustrated in the following graph.
The moderate climate in coastal areas is the result of the absorbing by water of huge amounts of solar heat energy during the day and the slow release of heat energy during the night. Inland areas typically experience much wider temperature extremes. The vast oceans on earth - about 75 percent of the surface area - are responsible for tempering the climate on Earth, permitting life to exist.
Heat capacity and latent heat are key properties that allow water - the oceans in particular - to play a major role in "regulating" Earth's climate. Water absorbs solar energy and releases it slowly; thus, larger bodies of water do not change temperature rapidly. Likewise, the high latent heat of vaporization indicates that when water vapour condenses into liquid droplets at high elevations (or high latitudes), the latent heat is released into the environment. It has the highest of all liquids' ability to conduct of heat.
Water has a strong attractive force that exists between molecules giving rise to a very high surface tension and therefore capillary forces. These capillary action refers to the tendency of water to move up a narrow tube against the forces are relied upon by all vascular plants, such as trees.
Because water molecules are not linear and the oxygen atom carries a slight negative charge, as distinct from the hydrogen atom - which is slightly positive charge means water is a polar molecule with an electrical dipole moment forming an unusually large number of inter-molecular hydrogen bonds, (4 of them), for a molecule of its size. Next to mercury, water has the highest surface tension of all commonly occurring liquids.
 Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
 Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
Surface tension is a manifestation of the presence of the hydrogen bond. Those molecules of water that are at the surface are strongly attracted to the molecules of water below them by their hydrogen bonds. If the diameter of the container is decreased, the combination of cohesion, which holds the water molecules together, and the adhesive attraction between the water molecules and the glass container will pull the column of water to great heights. Its surface tension is the highest of all liquids.
Water is a good solvent - often referred to as the universal solvent. Substances critically that dissolve in water, include oxygen and carbon dioxide. Dissolves more substances in greater quantities than any other liquid.
Water's geometry is such that the electrons are not uniformly distributed throughout the molecule. So the end of the molecule with greater electron density is slightly negative and the other slightly positive. Water and compounds like it are said to be polar, and kind of behave like a magnet. Water generally dissolves other substances that are also polar, but not non-polar substances like oil. Water is quite polar which explains many of its properties like its high melting and boiling points, high surface tension, and why it expands when frozen.
The balance of water on Earth remains constant over time, but individual water molecules can come and go, in and out of the atmosphere, snowfall, ice packs, glaciers, melt-waters, rainfall, springs, streams, aquifers, watering systems, rivers, ocean, back to the atmosphere, through the physical processes of evaporation, condensation, precipitation, infiltration, runoff, and subsurface flows. In doing this, water goes through different phases of solid, liquid, and gas.
The water cycle involves the exchange of heat energy, which leads to temperature changes. For instance, in the process of evaporation, water takes up energy from the surroundings and cools the environment. Conversely, in the process of condensation, water releases energy to its surroundings, warming the environment. The water cycle figures significantly in the maintenance of life and ecosystems on Earth. Even as water in each reservoir plays an important role, the water cycle brings added significance to the presence of water on our planet. By transferring water from one reservoir to another, the water cycle purifies water, replenishes the land with freshwater, and transports minerals to different parts of the globe. It is also involved in reshaping the geological features of the Earth, through such processes as erosion and sedimentation.
In addition, as the water cycle also involves heat exchange, it exerts an influence on climate as well. As the Earth's surface water evaporates, winds move water in the air from the sea to the land, increasing the amount of fresh water on land. Water vapour is converted to clouds that bring fresh water to land in the form of rain or snow (precipitation), falls on the ground, but what happens to that water depends greatly on the geography of the land at any particular place.
Last but not least it also has transparency to light, so some sunlight gets through the sea/ocean surfaces so that algae can photosynthesize in the first few metres of water at least.
Subscribe to:
Post Comments (Atom)










No comments:
Post a Comment